Revolutionary eye gene therapy gives children life-changing improvements
Four children have gained life-changing improvements in their sight following pioneering eye gene therapy at Great Ormond Street Hospital for a rare genetic blindness.
The revolutionary genetic treatment was delivered with experts from Moorfields Eye Hospital and University College London Institute of Ophthalmology, with the support of gene therapy company MeiraGTx.
The children were born with a severe genetic eye condition called Leber Congenital Amaurosis-4 (LCA4) which causes impairment to their sight due to a rare genetic deficiency that affects the 'AIPL1' gene. The condition means those affected are born with only sufficient sight to distinguish between light and darkness.
The gene defect causes the retinal cells in the eyes to malfunction and die, with children affected being legally certified as blind from birth. The new treatment is designed to enable the retinal cells to work better and to survive longer.
The procedure, developed by UCL scientists, consists of injecting healthy copies of the gene into the retina, at the back of the eye through keyhole surgery. These copies are put inside a harmless virus, which helps them to enter the retinal cells and replace the defective gene.
Published in The Lancet, the outcomes of the new treatment show that receiving the gene therapy at an early age can dramatically improve sight for children with this condition - one that is rare and particularly severe. These new findings offer hope that children affected by both rare and more common forms of genetic blindness may also benefit from genetic medicine in the future.
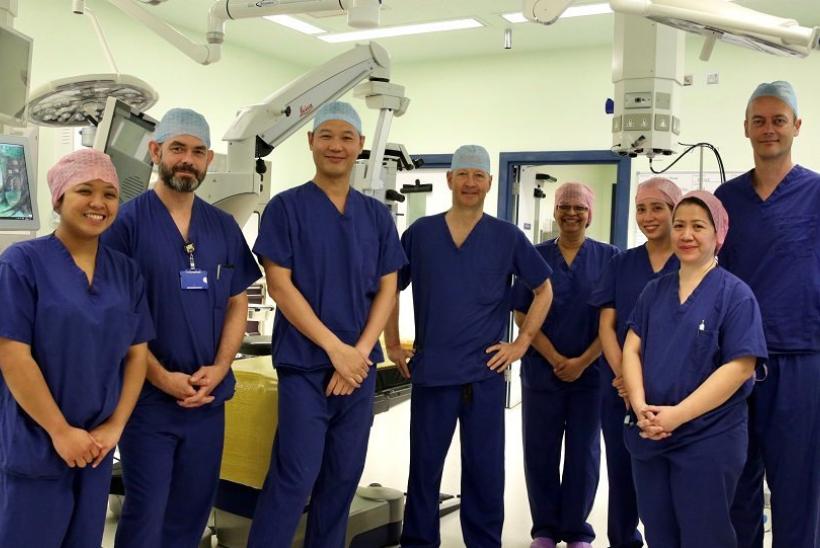
World-first surgery
Surgeons at GOSH and Moorfields Eye Hospital joined forces to deliver the revolutionary procedure on four patients. The children each received this novel therapy in one eye only, to ensure current level of sight in the other eye was preserved in case the treatment didn’t work or caused further decline. All four children saw remarkable improvements in the treated eye over the following three to four years, but lost sight in their untreated eye.
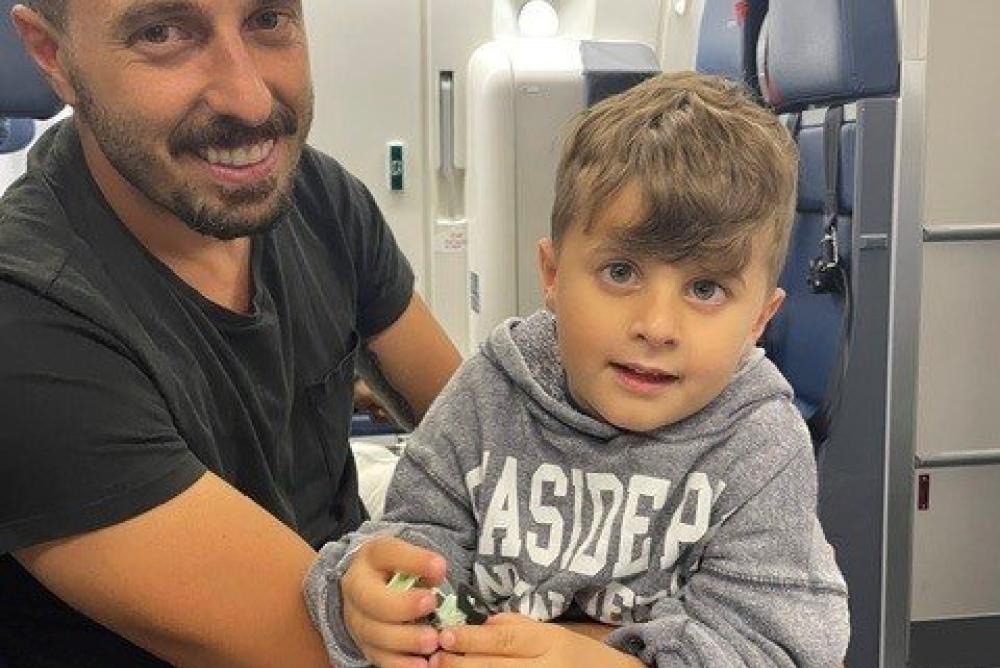
Six-year-old Jace, from Connecticut, USA, was one of the patients to receive the new treatment in October 2020.
Prior to surgery, Jace responded to light but was unable to track objects or see faces. His parents learnt about the gene therapy surgery at a LCA conference and later had discussions with clinicians at GOSH and Moorfields Eye Hospital about his suitability for the surgery.
Immediately after the surgery when Jace was two-years-old, his parents, said: “The team at GOSH that worked on Jace were incredible. We knew he was in the best hands under their care. Recovery has been going well and once we were able to remove the patch from his eye the morning after surgery, it has not seemed to bother him one bit. Our long term hope has been that the surgery helps to slow down the deterioration of his retina so that he can maintain his light perception. In taking part in this surgery and research, we also hope that this helps those studying the disease to learn more about LCA, specifically AIPL1.”
Speaking now, mum DJ added: “After the operation, Jace was immediately spinning, dancing and making the nurses laugh. He started to respond to the TV and phone within a few weeks of surgery, and within six months, could recognise and name his favourite cars from several metres away; it took his brain time, though to process what he could now see. Sleep can be difficult for children with sight loss, but he falls asleep much more easily now, making bedtimes an enjoyable experience.”
Chien Wong, consultant eye and retinal surgeon at GOSH, said: “This groundbreaking work epitomises collaboration in medicine at its best, with GOSH enabling surgeries in all four patients. Over the last few years, it’s been incredibly heart-warming to see how well the children have progressed and the life-changing impact the new treatment has given them and their families. This has exceeded my expectations.”
The research team is now exploring the means to make this new treatment more widely available.
Professor James Bainbridge, consultant retinal surgeon at Moorfields Eye Hospital and professor of retinal studies at UCL Institute of Ophthalmology, said: “Sight impairment in young children has a devastating effect on their development. Treatment in infancy with this new genetic medicine can transform the lives of those most severely affected.”
Professor Michel Michaelides, consultant retinal specialist at Moorfields Eye Hospital and professor of ophthalmology at the UCL Institute of Ophthalmology, commented: " We have, for the first time, an effective treatment for the most severe form of childhood blindness, and a potential paradigm shift to treatment at the earliest stages of the disease. The outcomes for these children are hugely impressive and show the power of gene therapy to change lives.”
The treatment was developed and manufactured at UCL under a manufacturing specials licence (MSL) held by UCL. MeiraGTx supported production, storage, quality assurance and dispensing under their MSL.
The procedure to administer the treatment to the affected children took place at Great Ormond Street Hospital. The children were assessed in the NIHR Moorfields Clinical Research Facility, and the NIHR Moorfields Biomedical Research Centre provided infrastructure for the research.
The work was partially funded by the National Institute for Health and Care Research (NIHR), MeiraGTx and Moorfields Eye Charity.