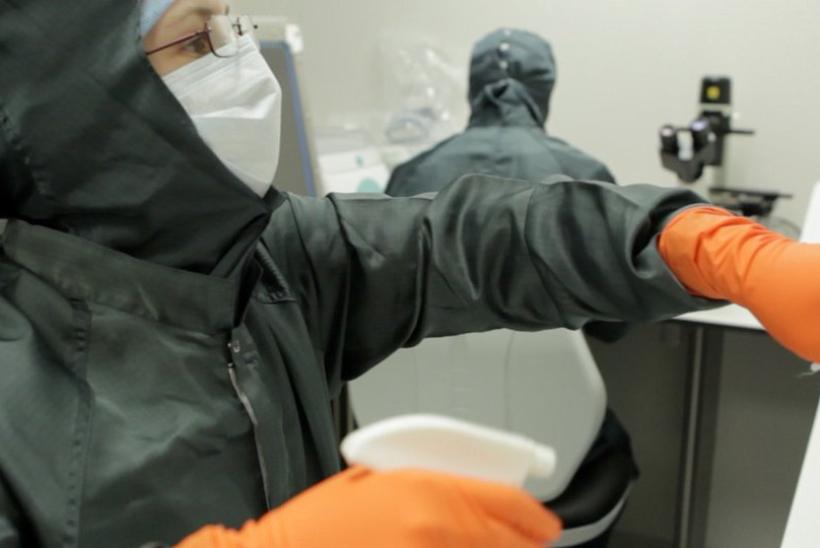
GOSH’s new facility for manufacturing cutting-edge gene and cell therapy products
GOSH recently opened new facilities for manufacturing cutting-edge gene and cell therapy products for clinical trials that can find new, personalised treatments for children with rare conditions.
The Cell and Gene Therapy Facility is a manufacturing site for advanced therapy medicinal products (ATMPs) licensed by the Medicines and Healthcare products Regulatory Agency. It is an invaluable resource for clinical trials and commercial partnerships, supporting rapid translation of cutting-edge research into clinical treatments for children with rare diseases.
The facility is comprised of 7 bespoke laboratories and provides comprehensive facilities for all stages of manufacture. This includes tissue culture labs, storage, waste treatment and quality control. This work is labour intensive, highly regulated and innovative. The facility also has a simulation suite that is used for education and training.
Cell and Gene Therapy products are manufactured specifically for each patient and often have a shelf-life of only a few hours, therefore manufacture on-site at Great Ormond Street Hospital (GOSH) can be crucial in delivering these treatments to patients.
The laboratories are based at the hospital and Zayed Centre for Research into Rare Disease in Children (ZCR) into Rare Disease in Children. The Centre, located next to GOSH, uniquely brings together pioneering research and clinical care under one roof to help drive forward new treatments and cures for children with rare diseases.
The work of the NIHR GOSH Biomedical Research Centre is also integral to facilitate research and development of cell and gene therapies produced at GOSH.
Ongoing projects
The Cell and Gene Therapy Facility supports production of ATMPs for a number of clinical trials. These include immunotherapy in multiple cancer types and enzyme replacement therapy Mucopolysaccharidosis type II (Hunter syndrome).
The facility provides a quality assured interface with industrial partners that require the manufacture of gene modified cell products for specific gene therapy studies, for example Virocell Biologics’ manufacturing of viral vectors for Phase 1 trials is based at this facility.
Gene and cell therapies are the cutting-edge in clinical research and development, holding promise to offer personalised treatment in rare diseases. The licensing of this new facility by the MHRA is a huge achievement for our Production and Quality teams who have set up and validated our processes and equipment. This state of the art facility significantly increases GOSH's capacity to delivery novel therapies in paediatric rare diseases.
Stephen Mathew, Head of Innovation at Great Ormond Street Hospital for Children