ICH researchers lead global team to develop a 3D bioprinting technique that could replace missing or damaged tissue without the need for open surgery
Using a minimally invasive technique dubbed ‘intravital 3D bioprinting’ scientists successfully created new muscle tissue in mice
A pioneering international study led by the UCL Great Ormond Street Institute of Child Health (ICH) has seen researchers develop a photosensitive bio-gel that uses light treatment to ‘print’ healthy new tissue directly into specific tissues and organs, and sustain blood supply that would allow it to thrive, according to results published in the Nature Biomedical Engineering journal today (22nd June).
In the muscle of live mice, the light-sensitive bio-gel acted as a type of bio-ink, effectively ‘printing’ 3D structures that supported the creation of muscle fibres. This new research, which was done in partnership with Great Ormond Street Hospital (GOSH), could pave the way for minimally invasive surgical techniques for organ repair and reconstruction that could remove the need for transplantation in children with complex conditions.
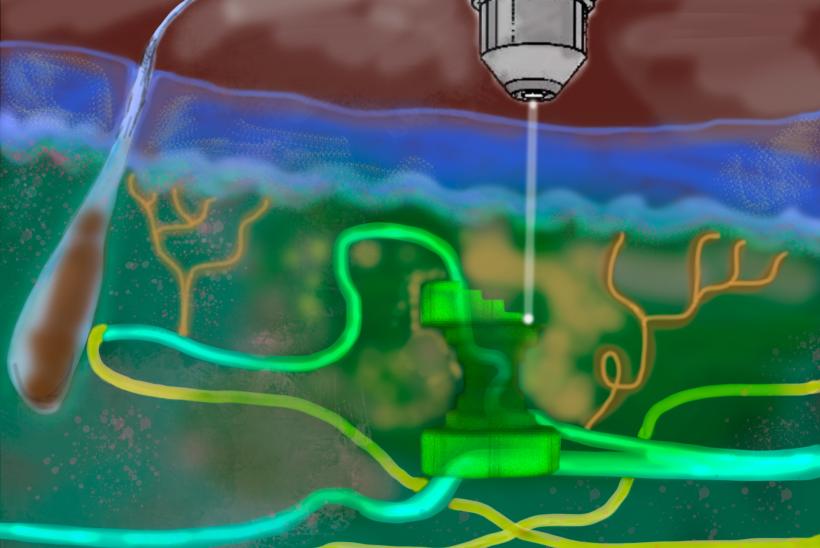
In the first step researchers loaded a liquid gel with cells carefully selected to suit the type of tissue being printed. The bio-gel was then injected into the area of interest in the body with a simple syringe. Once in place, the team directed a near infrared light at the area from outside the body. Polymers inside the bio-gel bonded together under this wavelength of light, printing (or solidifying) 3D structures layer by layer and allowing the cells to reach the desired position. With support from the structures, the cells adapted and connected to their new surroundings to form new tissue.
Initially tested on the skin and brain of the mouse, team’s technique – coined Intravital 3D Printing, or i3D Bioprinting – was also successfully carried out in the muscle of a mouse where it created new tissue without causing damage to surrounding organs or tissues. Furthermore, it did not create any waste material inside the body and has the potential to carry healthy donor cells. This could be life-changing in cases where a child’s own cells aren’t suitable or available to help repair or reconstruct damaged or missing tissue.
Project lead Professor Nicola Elvassore, whose research team spans across the ICH and centres in Italy and China, said: “Recent attempts at 3D bioprinting have required direct access to the tissue and space to manoeuvre the 3D bioprinting pen, to control how the tissue forms its shape and structure. That meant they focused on parts of the body that are easier to access, such as the skin. We’re really excited that our technique seems to be much more controllable in three dimensions, allowing us to accurately image in 3D the anatomical sites of interest and safely print new tissue in areas that aren’t easy to access without major surgery, like the brain.”
First-author Dr. Anna Urciuolo, visiting research associate in ICH and Junior PI in Italy, said: “It was an exciting and challenging project, which required the fusion of emerging technologies in a multidisciplinary approach. By performing 3D bioprinting directly within the body of live animal model, we were able to deliver donor muscle stem cells in a spatially controlled manner, increasing their ability to develop new muscle tissue.”
The international team, which included researchers from Italy’s University of Padova and Veneto Institute of Molecular Medicine, successfully ‘print’ the gel within skin, muscle and brain tissues.
Speaking about the medical implications, co-author Paolo De Coppi who is a Consultant at Great Ormond Street Hospital (GOSH) and Nuffield Professor of Surgery at ICH said: “This is an important step in repairing damaged tissue and offers the possibility of minimally invasive regeneration, which could change in the future the way we treat congenital malformations such as spina bifida and diaphragmatic hernia at GOSH .” He also added: “We still have plenty of work to do before we can safely use this approach with patients, but the findings are promising.”
Although still in its pre-clinical stage, this kind of tissue engineering research could lead to a new standard of care for patients with complex physical conditions, especially in the case of children with damaged organs.